-40%
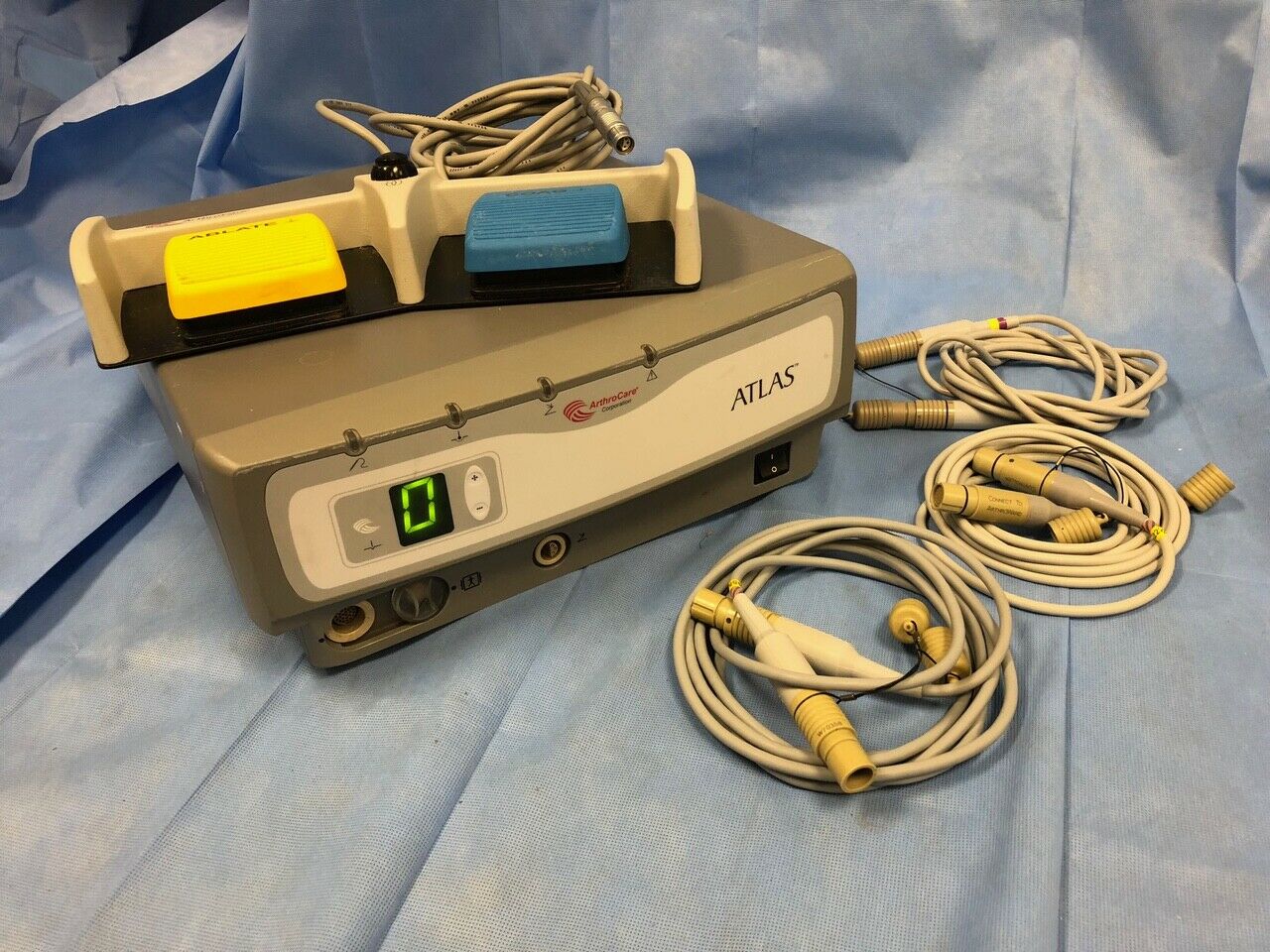
Arthrocare Atlas RF11000 w/ 10863 Footswitch & 3 Wand cables H0970-02 - Used
$ 198
- Description
- Size Guide
Description
1 - Arthrocare Atlas RF11000 console ...1 - Dual pedal Footswitch Part No.: 10863 ...
3 - Wand cables Part No.: H0970-02 ...
System powered on - no further testing able to be done.
Footswitch has rust on screw heads on bottom of unit.
Some of the Wand cables are missing a black ’O’ ring near end of connector that keeps safety cap on tight.
We don’t have the knowledge or ability to test these cables so they and the entire system and its components are being sold as is.
Overall cosmetic condition of the console is good with no cracks in front panel membrane.
Unit has minor scuffs, scratches, sticker residue, dirt, etc. needs to be cleaned and all components should be disinfected before being put into use.
It is up to the Purchaser to get a qualified Biomedical Engineer to test and evaluate this equipment to make sure that it meets manufacturer’s specifications and is safe for use for whatever intended purpose is.
We make new warranties expressed or implied.
Disclaimer
: This item is sold as is, no warranties implied. The item described and/or pictured offered here is in no way certified for, recommended for, or offered for any specific use. The purchaser agrees that the seller shall not be held responsible or liable for any injuries or damages, whether incidental or coincidental, associated in any way with this equipment. It is the sole responsibility of the purchaser to obtain qualified help to ensure the proper functioning, calibration, and safety (mechanical, electrical, etc.) of this equipment. The purchaser by biding on this item, indicates their knowledge of an agreement to the terms of this disclaimer. This item is sold completely as is/ as shown with no warranty either expressed or implied. The sale of this item maybe subject to regulations by the U.S. Food and Drug Administration, state and local regulatory agencies. If you should have questions about legal obligations, you should consult with the U.S. Food and Drug Administration.